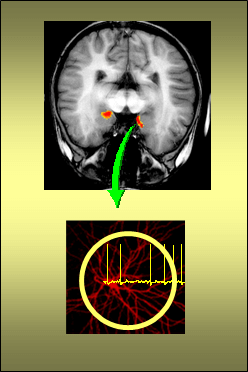
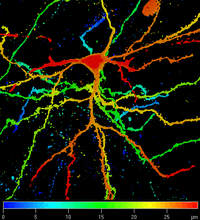
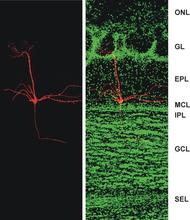
Faculty
FACULTY

Marjorie C. Gondre-Lewis, Ph.D.
She/Her
Professor of Neuroscience, Associate Dean for Faculty Development and Diversity
Anatomy
mgondre-lewis@Howard.edu
Byron D. Ford, PhD
He/him/his
Associate Dean of Research & Graduate Studies, Professor & Chair, Anatomy
Anatomy
byron.ford@howard.eduAngel Nkwain
She/Her
Research Coordinator & GHUCCTS Program Coordinator
Anatomy
Miranda Armour-Chelu, Assistant Professor, Ph.D., London University, United Kingdom, 1993.
Phone: (202) 806-6026, email: marmour-chelu@howard.edu
My research interests include systematics, ecomorphology, taphonomy and paleoecology of faunal assemblages dating from the middle Miocene to Recent. I have undertaken fieldwork in Hungary, Croatia, Jamaica, England and Abu Dhabi excavating fossil equids, bovids, proboscideans amongst other groups. My current research projects include Evolution of Central Paratethys (Hungary and Croatia) Miocene Vertebrate Communities, Systematics, Taphonomy and Paleoecology of African Equids (Laetoli, Olduvai, Tanzania and Sahabi, Libya). Publications
Raymond L. Bernor, Professor, Ph.D. University of California at Los Angeles, 1978.
Phone: (202) 806-4316, email: rbernor@comcast.net
My research interests since 1973 have been on the evolution of Eurasian and African Neogene and Quaternary mammal faunas. I have conducted fieldwork throughout Eurasia and Africa, but the last 15 years fieldwork has been principally in Central Europe, working on Middle and Late Miocene horizons of Germany (Hoewenegg, Germany), Hungary (Rudabanya, Baltavar and many other localities) and Croatia (broad survey of several Early and Late Miocene vertebrate localities; see attached PPT presentation). I have specialized in the systematics, functional anatomy, biogeographic and paleoecological reconstructions of whole mammal faunas, with particular interests in fossil equids, suids and hominoids. I am a member of the Steering Committee and leader of the Large Mammal working group of the Neogene Old World (NOW) Database directed by Professor Mikael Fortelius, Helsinki, Finland (http://www.helsinki.fi/science/now/). I am furthermore a member of the Revealing Human Origins Initiative Co-Directed by Professors F. Clark Howell and Tim White (University of California, Berkeley). Within this project I am involved in coordinating the Perissodactyl Research Group, I am a member of the Suid Research Group, and Co-Coordinator of the Program Informatics group (with John Damuth). My particular research interests within the RHOI are the reconstruction of equid and suid lineages within Africa, their paleoecologic contexts, and their phylogenetic and biogeographic relationships to Eurasian lineages. I am also interested in the biogeographic origin and paleoecologic context of the African ape-human clade. My current research is funded by the National Science Foundation and LSB Leakey Foundation. Past research has also been supported by the National Geographic Society, NATO, the American-Hungarian Fund and the Smithsonian Institution. My current paleontological field projects are at the late Miocene (10.3 m.y.) site of Hoewenegg (Hegau, Germany), and Lucane, Croatia. Publications
Antonei Csoka, Assistant Professor, Ph.D.,
Dr. Csoka received his B.S. in Genetics from the University of Newcastle, U.K., his M.Sc. in Molecular Pathology and Toxicology from the University of Leicester, U.K., and his Ph.D. in Cell and Molecular Biology from the University of Debrecen, Hungary. He has performed postdoctoral research at the University of California, San Francisco, where he cloned the human hyaluronidase genes, which are involved in fertilization, embryonic development, and cancer. As a postdoctoral research associate at Brown University, Dr. Csoka was instrumental in the identification of the gene that causes Hutchinson-Gilford Progeria Syndrome (progeria), a disease with many features of “accelerated aging.” It is hoped that the identification of the gene for progeria will provide insights into the mechanisms of normal aging. As an assistant Professor in the Department of Anatomy at Howard University, Dr. Csoka is developing animal models of progeria, studying the role of nuclear lamina dysfunction in human aging, and investigating the potential of induced pluripotent stem cells, cellular reprogramming and epigenetic rejuvenation for the treatment of age-related diseases.
Rui Diogo, Assistant Professor, Ph.D., University of Liege, Belgium, 2003 & George Washington University, 2011.
Phone: (202) 651-0439, email: rui.diogo@howard.edu
I am an Advisory Board Member of the Journal "Progress in Fish Research" (Gwalior, India), a Consortium Member of the "Timetree" project (book + website: www.timetree.org), an Editorial Board Member of the "Open Anatomy Journal", and a Member of the American Association of Anatomists.
My research is mainly focused on:
- Primate Comparative Anatomy
- Primate Phylogeny and Evolution
- Chordate Comparative Anatomy
- Chordate Phylogeny and Evolution
- Chordate Developmental Biology
- Chordate Functional Morphology
- Philosophy, History and Biases of Science
For more details about my research interests, my CV, my lab, and my publications, please see: ruidiogolab.com. Publications
Edwin Gilland, Associate Professor, Ph.D.
Phone: (774) 521-9229, email: egilland@howard.edu Publications
Marjorie Gondre-Lewis, Professor, Ph.D., Albert Einstein College of Medicine; PostDoc, National Institute of Child Health and Human Development
Phone: (202) 806-5274, email: mgondre-lewis@howard.edu
Dr. Gondré-Lewis is Director of the Developmental Neuropsychopharmacology Laboratory at the Howard University College of Medicine. Her research team has a dual focus to investigate environmental factors that influence brain development and maturation, and also to investigate mechanisms of reward associated with escalation of drug use, especially excessive alcohol consumption and opioid addiction. These research foci converge in that many experiences of early life adversity, which disrupt neural circuits in distinct anatomical sites, also lead to neuropsychiatric disorders with altered cognition, mood/affect, and reward/addiction. The laboratory tests various pharmacological, genetic, and biochemical interventions to restore control behaviors and reduce the manifestation of those associated with depression, anhedonia, anxiety, addiction, impulsivity, fear learning. Human correlates are Attention Deficit and Hyperactivity Disorder (ADHD), Major Depressive Disorder (MDD), Generalized Anxiety Disorder (GAD), Schizophrenia, Substance Use Disorders (SUD),
Dr. Gondré-Lewis has served as a reviewer and is on the editorial board for many journals, on several grant review panels for the National Institutes of Health (NIH), and shares her work internationally. She is an author of numerous research articles, reflective of her broad background in developmental brain disorders and mechanisms of drugs of abuse. She has investigated disorders like Smith-Lemli-Opitz syndrome, Niemann-Pick disease, and other genetic brain diseases. She now focuses on both genetic and epigenetic (environmental experience) modulators of brain development and maturation, and how drugs impact this development. The laboratory has received funding from NIAAA, NIMHD, NINDS, NIGMS, NSF, as well as internal and corporate sources.
Main Research Topics are aimed at:
- Uncovering immediate and long-term behavioral, neuroanatomical and molecular disruptions caused by the experience of early life adversity and developmental drug exposure (nicotine) during prenatal life, infancy and adolescence.
- Investigating molecular bases of alcohol addiction and co-morbid neuropsychiatric disease as mediated by neuroinflammation, CRF, GABA, and other molecular circuits.
- Testing small molecules and natural products that target the reward pathway to improve cognition and affect in rodent models of stress and addiction.
- Targeting the genetic bases for opioid use disorder to develop effective treatments to restore dopamine homeostasis in humans—consideration for psychosocial determinants of brain health.
Selected Publications: For fuller list, search Pubmed for “Gondre-Lewis”
- Balan I, Warnock KT, Puche A, Gondré-Lewis MC, Aurelian L. Innately activated TLR4 signal in the nucleus accumbens is sustained by CRF amplification loop and regulates impulsivity. Brain Behav Immun. 2017 Nov 13. pii: S0889-1591(17)30509-3.
- Gondré-Lewis MC, Warnock KT, Wang H, June HL Jr, Bell KA, Rabe H, Tiruveedhula VV, Cook J, Lüddens H, Aurelian L, June HL Sr. Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism. Stress (Amsterdam, Netherlands). 2016; 19(2):235-47. NIHMSID: NIHMS803288
- Blum K, Badgaiyan RD, Dunston GM, Baron D, Modestino EJ, McLaughlin T, Steinberg B, Gold MS, Gondré-Lewis MC. The DRD2 Taq1A A1 Allele May Magnify the Risk of Alzheimer's in Aging African-Americans. Mol Neurobiol. 2017 Sep 30. doi: 10.1007/s12035-017-0758-1.
- Tao R, Davis KN, Li C, Shin JH, Gao Y, Jaffe AE, Gondré-Lewis MC, Weinberger DR, Kleinman JE, Hyde TM. GAD1 alternative transcripts and DNA methylation in human prefrontal cortex and hippocampus in brain development, schizophrenia. Mol Psychiatry. 2017 May 9. doi: 10.1038/mp.2017.105.
- Arvaniti M, Jensen MM, Soni N, Wang H, Klein AB, Thiriet N, Pinborg LH, Muldoon PP, Wienecke J, Imad Damaj M, Kohlmeier KA, Gondré-Lewis MC, Mikkelsen JD, Thomsen MS. Functional interaction between Lypd6 and nicotinic acetylcholine receptors. Journal of neurochemistry. 2016; 138(6):806-20. NIHMSID: NIHMS798679
- Wang H, Gondré-Lewis MC. Prenatal nicotine and maternal deprivation stress de-regulate the development of CA1, CA3, and dentate gyrus neurons in hippocampus of infant rats. PLoS One. 2013 Jun 13;8(6):e65517. doi: 10.1371/journal.pone.0065517. Print 2013.
* For earlier work, search Pubmed for “Gondre”
Thomas Heinbockel, Professor, Ph.D.,
University of Arizona, 1997.
Phone: (202) 806-9873, email: theinbockel@howard.edu
https://profiles.howard.edu/profile/34601/thomas-heinbockel
Neurobiology of Olfactory and Limbic Systems
Research in my lab is aimed at elucidating organizational principles of neural systems in the brain using electrophysiological, pharmacological and anatomical methods. In particular, we are interested in the functional organization of the limbic and olfactory system.
Objectives and Questions in Neuroscience:
-Structural and functional organization of the olfactory & limbic system (hippocampus, amygdala)
-Neural mechanisms underlying cellular signaling in these systems; synaptic plasticity, learning, memory
-Sensory or synaptic information processing and integration in the brain
-Molecular and cellular changes during neurological and neuropsychiatric disease states
-Discovery and Development of anticonvulsant drugs
-Epigenetic effects of drugs of abuse on gene expression profiles
-Translational neuroscience
Synaptic plasticity and neuromodulation
In the limbic system our research activities have been centered on a subcortical structure of the vertebrate brain, the amygdala, a key brain site for emotion, fear, learning and memory. The amygdala is essential for developing an inner view of the outside sensory world and is thought to be one of the key structures for the interpretation of sensory information associated with motivation and emotion. The main goal of this line of work is to reveal functions of the amygdala at the cellular and network level and to integrate this knowledge into the functioning of higher order brain systems. Research on the amygdala includes topics such as synaptic transmission, neuromodulation, synaptic plasticity as well mechanisms for generating rhythmic and epileptiform activity.
Sensory coding and olfactory processing
Olfaction is a fundamental sensory modality and provides us with some of our emotionally most stimulating and lasting memories, yet many of the mysteries of this modality, from the molecular to the systems level, still remain unknown. In addition, the olfactory pathway is unique in sending sensory information directly to the cortex and areas involved in emotion and memory (amygdala, limbic areas), and bypassing the thalamus as an intermediary step.
Intrinsic and synaptic properties of olfactory bulb neurons
In a rodent olfactory bulb slice preparation my lab uses patch clamp electrophysiology and imaging techniques to characterize biophysical, cellular and synaptic properties of neurons. The olfactory bulb is the first central relay station for olfactory information conveyed from the nasal epithelium by olfactory receptor neurons. The olfactory bulb contains output neurons (mitral and tufted cells) that transmit olfactory information to higher order olfactory structures and to other brain systems. The relay from the nose to mitral and tufted cells is strongly regulated by local intrabulbar circuitry, including inhibitory granule cells, and by centrifugal inputs to the olfactory bulb from other parts of the brain. Questions that we address include: How do intrinsic and synaptic neuronal properties relate to information coding and neural network function of this system? What is the functional role of different neurotransmitter receptors (ionotropic and metabotropic) on these cell types? How do the various neuromodulator systems within the olfactory bulb and from centrifugal fibers shape the response to olfactory nerve input, as well as synaptic output to higher brain centers? My research on both the olfactory and limbic system has been directed at understanding mechanisms of information processing that form the basis of persistent functional changes in these systems and their relation to neurological and neuropsychiatric disorders.
Heinbockel Lab - Involvement of Students at all Levels in Research, Presentations and Publications
Heinbockel Lab - Ongoing Projects
- Funded by NIH, NSF and private foundations
- Support for students: undergrad., postbac, graduate, postdocs
- Continuously publishing in high profile journals
- In the lab, we use an array of techniques in neurophysiology, neuroanatomy, and neuropharmacology:
- patch clamp recording, intra- and extracellular electrophysiology
- voltage-sensitive dye imaging, intracellular calcium measurements
- microstimulation, microinjection, microsurgery
- intracellular staining, neuronal tracing,
- histochemistry, immunocytochemistry
- light and laser scanning confocal microscopy
- photolysis of caged compounds
- differential interference contrast visualization of cells in living slices
- microarray
Laboratory Personnel
The laboratory has openings for technicians, graduate students, and postdoctoral fellows. Please email your CV and letter of interest.
James Steven Wilson, Associate Professor, Ph.D., Medical College of Virginia, 1978.
Phone: (202) 806-6559, email: jwilson@howard.edu
My laboratory is studying the causes and possible cures of Parkinson's disease, a major neurological disorder affecting primarily the elderly. Recently, a major advancement was made in this field with the discovery of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which produces a Parkinsonian-like syndrome when injected into certain laboratory animals. Using this selective neurotoxin, we are studying at the light and electronmicroscopic levels the spatio-temporal process of neural degeneration. These studies have provided important insights into the organization of the nigrostriatal system (the area damaged in idiopathic and MPTP-induced Parkinson's disease) and the mechanism of MPTP's selective toxicity. We are also studying the physiological effects of MPTP with the hopes of understanding the function of the nigrostriatal system and its neurotransmitter, dopamine, in normal and pathological brains.
Janine Ziermann, Assistant Professor, Ph.D
1319 Numa P.G. Adams Bldg
Website: https://jm13ziermann.wixsite.com/jmzlab
Howard University faculty webpage: https://profiles.howard.edu/profile/40426/janine-m-ziermann
E-mail: Janine.ziermann@howard.edu
Howard University College of Medicine
520 W St NW, Numa Adams Building Room 1319
Washington, DC, 20059
Janine M. Ziermann, PhD, is an Assistant Professor at the Howard University
College of Medicine in Washington, DC. She received her PhD in Germany
studying the evolution and development of head muscles in larval amphibians.
This was followed by a postdoc in the Netherlands and one in the USA to
further study vertebrates. Her research focuses on the evolution and development of the
cardiopharyngeal field, a field that gives rise to head, neck, and heart
musculature. Additionally, she aims to use the knowledge from her research
to understand the pathology of congenital defects, which often affect both
head and heart structures. She won several awards, including the ‘American Association of Anatomists
(AAA) and the Keith and Marion Moore Young Anatomist’s Publication Award’
(YAPA). Furthermore, she single authored or co-authored more than 30 papers
in top journals such as Nature, Biological Reviews, books, book chapters, and
commentaries. https://www.researchgate.net/profile/Janine_Ziermann
Selected Publications
ZIERMANN, Janine M.; DIOGO, Rui; NODEN, Drew M. Neural crest and the patterning of vertebrate
craniofacial muscles. Genesis, 2018, e23097.
ZIERMANN, Janine M., et al. Cephalic muscle development in the Australian lungfish, Neoceratodus
forsteri. Journal of Morphology, 2018, 279.4: 494-516.
ZIERMANN, Janine M.; FREITAS, Renata; DIOGO, Rui. Muscle development in the shark Scyliorhinus
canicula: implications for the evolution of the gnathostome head and paired appendage musculature.
Frontiers in Zoology, 2017, 14.1: 31.
DIOGO, Rui, et al. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature, 2015,
520.7548: 466.
ZIERMANN, Janine M.; DIOGO, Rui. Cranial muscle development in frogs with different developmental
modes: direct development versus biphasic development. Journal of Morphology, 2014, 275.4: 398-413.
Shaolin Liu, Assistant Professor, PhD, Peking Union Medical College, Postdoc, Yale University and Case Western Reserve University.
Phone: 202-806-3851; Email: shaolin.liu@howard.edu
Current research in my lab focuses on how sensory information is processed at the cellular and circuit levels with a model of the mouse olfactory system, a highly conservative sensory modality with output directly to the limbic system and heavy involvement in emotion, learning and memory in humans. Specifically, our present research project addresses fundamental questions on the anatomical and functional organization of neural pathways in the olfactory systems by capitalizing on cutting-edge technologies, including cell type-specific opto- and chemo-genetics combined with in vitro and in vivo electrophysiology, in vivo calcium imaging, immunohistochemistry, confocal imaging as well as behavioral approaches. Our work is presently supported by federal fund from NIH and has led to important findings published in high-profile peer-reviewed journals.
Ongoing and upcoming research also includes neuromodulation of information processing in the limbic system with particular concentration on the amygdala and entorhinal cortex, both of wihch receive direct synaptic input from the olfactory bulb and are heavily targeted by serotonergic, cholinergic, and dopaminergic modulation. Dysfunction of each of these neuromodulatory systems is involved in a series of neurological and neuropsychiatric disorders including anxiety, depression, Schizophrenia, Autism, Alzheimer's disease (AD), Parkinson's disease (PD), many of which have olfactory deficit as their early stage symptom. Thus, understanding the mechanisms of olfactory dysfuntion in these disease conditions is of importance to human health as it potentially helps us develop approaches for early diagnosis and pinpoint the underlying physiological and pathophysiological mechanisms. Our goal is to unveil the organizational and functional mechanisms underlying signal processing in these limbic subregions and how neuromodulation contributes to olfactory dysfunction and other behavioral abnormalities in these neurological or neuropsychiatric disorders by capitalizing on our cutting-edge multidisciplinary approaches and transgenic and/or pharmacological animal models.
Selective Publications
We have published peer-reviewed articles in the following journals.
- Liu X & Liu S#. (2018) Cholecystokinin selectively activates short axon cells to enhance inhibition of olfactory bulb output neurons. J Physiol, 596: 2185-2207.
- Liu S#, Puche AC, Shipley MT. (2016) The interglomerular circuit potently inhibits the olfactory bulb output neurons by both direct and indirect pathways. J Neurosci 36: 9604-9617.
- Liu S#, et al. (2015) Muscarinic receptors modulate dendrodendritic inhibitory synapses to sculpt glomerular output. J Neurosci 35:5680-5692.
- Liu S#, Plachez C, Shao Z, Puche AC, & Shipley MT (2013) Olfactory bulb short axon cell release of GABA and dopamine produces a temporally biphasic inhibition-excitation response in external tufted cells. J Neurosci 33:2916-2926.
- Liu S, Aungst JL, Puche AC, & Shipley MT (2012) Serotonin modulates the population activity profile of olfactory bulb external tufted cells. J Neurophysiol 107:473-483.
- Liu S & Shipley MT (2008) Multiple conductances cooperatively regulate spontaneous bursting in mouse olfactory bulb external tufted cells. J Neurosci 28:1625-1639.
- Liu S & Shipley MT (2008) Intrinsic conductances actively shape excitatory and inhibitory postsynaptic responses in olfactory bulb external tufted cells. J Neurosci 28:10311-10322.
- Liu S, Rao Y, & Daw N (2003) Roles of protein kinase A and protein kinase G in synaptic plasticity in the visual cortex. Cereb Cortex 13:864-869
Sulman Rahmat, Ph.D, Assistant Professor
I received a Ph.D. in Anatomy from the Department of Anatomy at the Howard University College of Medicine (HUCOM) in Washington D.C. My dissertation was titled “Hindbrain Neurovascular Anatomy of Goldfish and Zebrafish, with a Comparative Analysis of Brainstem Vasculature in Vertebrates” and was prepared under the tutelage of Dr. Edwin Gilland. My research involved vascular injections and microdissection of the brains of numerous vertebrate taxa in order to demonstrate brainstem vascular supply and evaluate the blood supply to neurons in hindbrain serial sections. Numerous imaging techniques were utilized, including fluorescent confocal microscopy and microCT. I expanded my dissertation research into the most complete survey on vertebrate hindbrain vasculature to date, examining the carotid-basilar arterial tree and hindbrain penetrating branches (Rahmat and Gilland, 2014).
My current research focuses on developmental and evolutionary biology, using my background in anatomy and neuroanatomy. I am interested in: 1) the neuronal and vascular development of the brain in vertebrates, working primarily on the model teleosts goldfish and zebrafish; and 2) correlating the osteology of seals (fossil and modern) and carnivorans (terrestrial and marine) with ecological, biogeographical and morphological implications. I am involved in collaborative studies with scientists within Howard University (Drs. Gilland, Heinbockel, and Koretsky) and outside of Howard (multiple collaborators from Marine Biological Laboratory, Woods Hole Oceanographic Institute, New York University Langone Medical Center, Cornell University, Smithsonian National Museum of Natural History, Calvert Marine Museum and American Museum of Natural History).
Despite a long history of anatomical studies, the vascular organization of the brainstem is poorly known in most vertebrates. My research examines results from the past 150 years and compares them with a series of new vascular micro-dissections to provide an overall assessment of vertebrate hindbrain vasculature. Although there are obvious differences in vertebrate body size and habitats, I was able to demonstrate a highly conserved suite of hindbrain vascular features in a wide range of taxa. My thesis and the initial journal article stemming from it are the first publications to demonstrate fully that the neurovascular pattern in teleost fish is unique among vertebrates and likely constitutes a fundamental adaptive innovation contributing to the immense success of this group. The series of midline segmental central arterial stems arising from the basilar artery and the intramural vascular branching pattern within the sub-ependymal zone of the hindbrain found in teleosts points to new areas of neuro-anatomical and embryological studies that I am currently pursuing. As a result, I currently am the Principal Investigator (PI) on an extramural grant examining the neuronal and vascular anatomy of the goldfish hindbrain. In addition, I am the PI on an NSF Grant submission (currently under review) that will use transgenic zebrafish lines, with fluorescent neurons, circulating blood cells and endothelial cells, to define and reconstruct larval and adult zebrafish neuronal and vascular anatomy in two areas of the brain with clear behavioral and structural features: the olfactory bulb and hindbrain. Once the neurovascular anatomy has been mapped, I plan to perform future studies to demonstrate the regulation of blood flow during neuronal activity in these two areas in zebrafish, using various odorant stimulants for the olfactory bulb and anoxic/hypoxic stimuli for hindbrain respiratory centers.
My collaborative work on fossil true seals (Phocidae) includes identifying the first record of fossil Cystophorine seals with obvious pachyosteosclerotic bones, including naming a new genus (Pachyphoca) with two new species (P. ukrainica and P. chapskii). I also described a new monachine seal genus (Terranectes) and two new species (T. magnus and T. parvus) from fossil finds off the coast of the Chesapeake Bay. I am the PI on another extramural grant examining pachyosteosclerosis in seals. I have published studies on the comparative examination of mandibular morphology in fossil and Recent seals, marine mammals and some terrestrial carnivores and an extensive morphological review focusing on the origin and dispersal of seals. My findings have improved the classification of true seals and have enhanced knowledge of general morphology. Future projects I plan to pursue include the first comparative description of inner ear structures of terrestrial and marine carnivorans, and the first detailed study of the neurocranium of seals. The cladistic, morphological and morphometric analyses in my studies have resulted in emended diagnoses and redescriptions to clarify phylogenetic relationships within Phocidae.
My comparative anatomy research, covering not only neurovascular anatomy of transgenic zebrafish, sectioned goldfish, and a broad range of vertebrates, but also the musculoskeletal morphology of terrestrial and marine carnivorans, provides me with an expansive background to work in numerous fields.
Dr. Rahmat Publications
PEER-REVIEWED ARTICLES, PUBLISHED/ACCEPTED
2018 Sulman J. Rahmat, Fernando Muniz, Antonio Toscano, Raúl Esperante and Irina Koretsky. First European record of Homiphoca (Phocidae:Monachinae:Lobodontini) and its bearing on the paleobiogeography of the genus. Historical Biology: International Journal of Paleobiology. DOI: 10.1080/08912963.2018.1507030
2018 Sulman J. Rahmat and Irina Koretsky. New findings of early Middle Miocene fossil for the extinct subfamily Devinophocinae from Vienna Basin. Vestnik Zoologii. (accepted for publication)
2017 Irina Koretsky and Sulman J. Rahmat. Preliminary report of pachyosteosclerotic bones in seals. Open Access Research in Anatomy. 1:1-3. OARA.000501.2017.
2017 Sulman J. Rahmat, Irina Koretsky, Jason E. Osborne and Aaron A. Alford. Miocene Seals from the Western Shore of the Chesapeake Bay. Vestnik Zoologii. 51(3): 221-242.
2016 Irina Koretsky, Larry Barnes and Sulman J. Rahmat. Re-evaluation of morphological characters questions current views of pinniped origins. Vestnik Zoologii. 50(4):327-354.
2016 Sulman J. Rahmat and Irina Koretsky. First record of postcranial bones in Devinophocaemryi (Carnivora, Phocidae, Devinophocinae). Vestnik Zoologii. 50:71-84.
2015 Sulman J. Rahmat and Irina Koretsky. Diversity of mandibular morphology in some carnivorans. Vestnik Zoologii. 49:267-284.
2015 Irina Koretsky and Sulman J. Rahmat. A New Species of the Subfamily Devinophocinae (Carnivora:Phocidae) from the Central Paratethys. Rivista Italiana di Paleontologia e Stratigrafia, 121(1):1-17.
2015 Irina Koretsky, Noud Peters and Sulman J. Rahmat. New findings of Praepusa (Carnivora, Phocidae, Phocinae) from the Central Paratethys supports east to west Neogene dispersal of true seals. Vestnik Zoologii. 49(1):457-466.
2014 Sulman J. Rahmat, Edwin Gilland, and Thomas Heinbockel. Maturation of vascular anatomy in the zebrafish brain. The FASEB Journal. Vol.28, No.1, Suppl.726.1.
2014 Irina Koretsky, Sulman J. Rahmat and Noud Peters. Rare Late Miocene Seal Taxa(Carnivora, Phocidae) from the North Sea Basin. Vestnik Zoologii, 48(5): 419-432.
2014 Irina Koretsky, Sulman J. Rahmat and Noud Peters. Remarks on Correlations and Implications of the Mandibular Structure and Diet in some Seals. Vestnik Zoologii, 48(3):255–268.
2014 Sulman J. Rahmat and Edwin Gilland. Comparative Anatomy of the Carotid-Basilar Arterial Trunk and Hindbrain Penetrating Arteries in Vertebrates. The Open Anatomy Journal. 6:1-26.
2013 Irina Koretsky and Sulman J. Rahmat. First Record of Fossil Cystophorinae (Carnivora, Phocidae): Middle Miocene Seals from the Northern Paratethys. Rivista Italiana di Paleontologia e Stratigrafia. 119:325-350.